Refining iron into steel involves a process of removing impurities like carbon, silicon, sulfur, and phosphorus from raw iron (usually pig iron) and carefully controlling the alloying elements to achieve desired properties. Here’s an overview of the process:

- Raw Material Preparation
Pig Iron: Obtained from a blast furnace, pig iron contains about 3-4% carbon and various impurities.
Scrap Steel: Often added as a recycled material to control carbon content and reduce costs.
- Primary Steelmaking Processes
Steel is produced by reducing the carbon content in pig iron. The most common methods are:
a. Basic Oxygen Furnace (BOF):
Input: Pig iron, scrap steel, and fluxes (like lime or dolomite).
Process:
- Oxygen is blown into the molten iron at high speeds.
- Carbon reacts with oxygen to form carbon monoxide (CO) and carbon dioxide (CO₂), reducing carbon content.
- Impurities like sulfur and phosphorus are removed via chemical reactions with the flux.
Output: High-quality steel.
b. Electric Arc Furnace (EAF):
Input: Primarily scrap steel, with some pig iron or direct-reduced iron (DRI).
Process:
- An electric current melts the scrap in the furnace.
- Alloying elements are added to achieve desired steel properties.
Output: Steel, often used for specialty products.
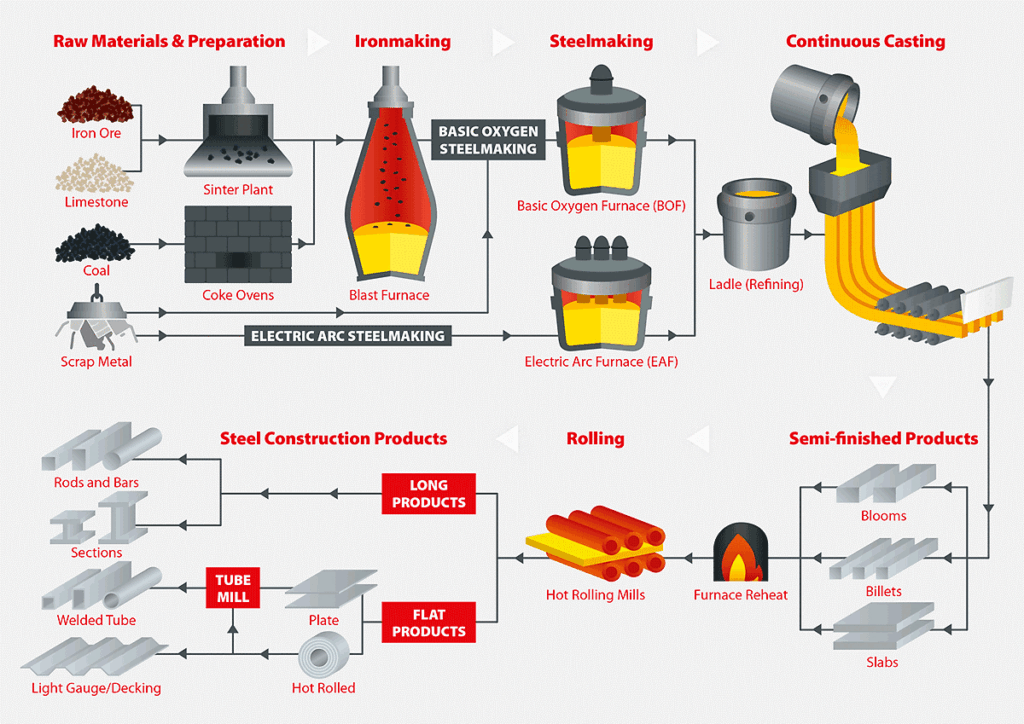
- Secondary Refining
To achieve specific properties and purity levels, molten steel undergoes further treatment:
Degassing: Removes dissolved gases like hydrogen, oxygen, and nitrogen.
Alloying: Elements such as chromium